
The fifth electron is added to a 2p orbital, the sublevel next higher in energy (Figure 5.9). Four electrons fill both the 1s and 2s orbitals. Therefore, the electron configuration of lithium is:īoron (atomic number 5) has five electrons. In order to write its electron configuration, we must first determine (from Figure 5.9) that the 2s sublevel is next higher in energy after the 1s sublevel. The element lithium (atomic number 3) has three electrons. Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have exactly the same energy as the single hydrogen electron, even though all are in the 1s sublevel. Two electrons completely fill the first energy level. Therefore, the electron configuration of hydrogen is written:įor helium (atomic number 2), which has two electrons, the electron configuration is: The single electron is assigned to the 1s sublevel, the lowest-energy sublevel in the lowest-energy level. The order is summarized under the diagram.įIGURE 5.9 The arrow shows a second way of remembering the order in which sublevels fill.Īn atom of hydrogen (atomic number 1) has one proton and one electron. To use this figure, read along the diagonal lines in the direction of the arrow. The principal energy levels are listed in columns, starting at the left with the 1s level.
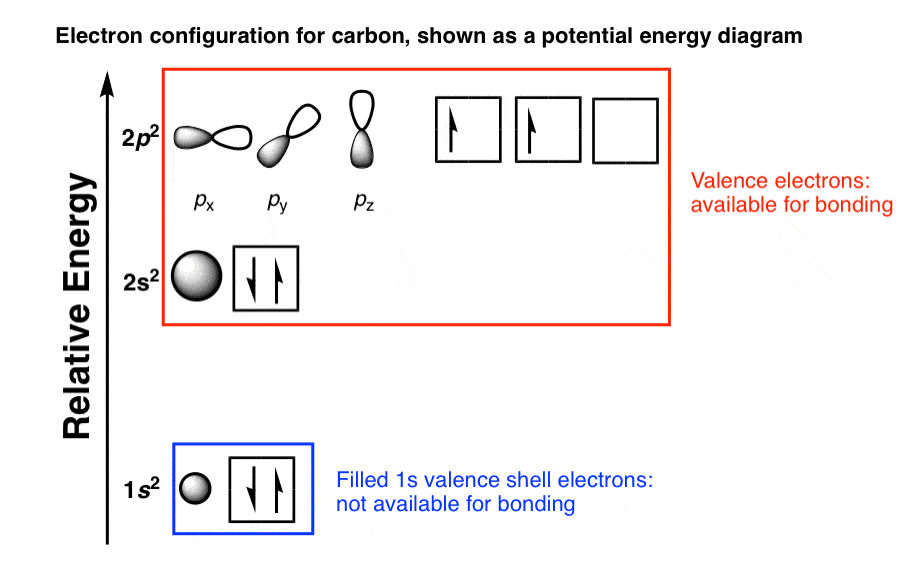
This order is difficult to remember and often hard to determine from energy-level diagrams such as Figure 5.8Ī more convenient way to remember the order is to use Figure 5.9. The first sublevel filled will be the 1s sublevel, then the 2s sublevel, the 2p sublevel, the 3s, 3p, 4s, 3d, and so on. Each added electron is assigned to the lowest-energy sublevel available. To determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of electrons equals the number of protons in the nucleus. This is the reason that carbon is different from the silicon and both of them are different from other elements of this group.The electron configuration of an atom shows the number of electrons in each sublevel in each energy level of the ground-state atom. The penultimate shell of carbon contains the s 2 electrons, silicon has s 2p 6 electrons and germanium contains the s 2p 6d 10 electrons and is unsaturated. So, they have an electronic configuration of s 2p 2 in their valance shell. Two of the electrons are in the s orbital and the remaining two are in the p orbital. The electronic configuration of group 14 elements shows that these elements have four electrons in their valance (ultimate shell). The electronic configuration for each element of this group is given below. There are two electrons in the outermost p orbitals of these elements. The general electronic configuration of group 14 elements is ns 2np 2. It is due to the fact that orbitals specify the position and shape of the regions of the space that is occupied by the electrons. Orbitals and electrons are very closely related however, orbitals provide a much accurate picture of the electronic configuration of an atom. Each column in the periodic table reflects the number of the electrons in the valance shells of each of the element which in turn strongly determine how the element will react. Typically, the sharing of electrons is observed to complete the valance shells by forming the multiple bonds with the other atoms. Carbon group the group 14 elements have four electrons in the outer shell. In neutral atoms, the number of electrons is equal to the number of the protons and atomic number can be easily determined by using the electron number. In the periodic table, elements are placed according to their atomic numbers that how many protons they have. Hund’s rule states that the pairing of electrons in the orbital only takes place when there is one electron in each subshell. The Pauli exclusion principle states that all four quantum numbers for any of the two electrons in an atom can never be the same. Another rule is defined by the Pauli and he defined a set of the unique quantum numbers for each of the electron. So, electrons fill the energy levels as defined by Aufbau’s principle. Electrons fill the orbitals of the atom in such a way that the energy of the atom is at the minimum level.


 0 kommentar(er)
0 kommentar(er)
